ImmuneMed announced on Monday that it will go into phase II clinical trials in South Korea and overseas regarding antiviral candidate “hzVSF” for COVID-19 treatment starting from next month.
“We expect that we will receive an approval for phase II clinical trials (investigational new drug (IND)) from the Ministry of Food and Drug Safety (MFDS) sometime during next month.” said CEO Kim Yoon-won of ImmuneMed. “As soon as we receive an approval, we will start conducting phase II trials on COVID-19 patients who are currently in a critical or serious stage.”
ImmuneMed was planning to start phase II trials back in July initially. However, it received a request from the MFDS regarding supplementing its plan on clinical trials and has been supplementing its clinical trials plan.
The company is also expected to go through phase II trials in other countries as well shortly. It is currently preparing to go through phase II trials in the U.S. while receiving a support from a multinational pharmaceutical service company called EVERSANA. It will look to receive an approval for IND by the end of this month and conduct phase II trials on about 110 patients starting from early next year. To do so, it is planning to hold a pre-IND meeting with the Food and Drug Administration.
In addition to the U.S., it is preparing to go through phase II trials in Italy, Russia, and Indonesia sometime during next month. Its goal is to receive approvals from these countries on emergency use of “hzVSF” by the first half of 2021.
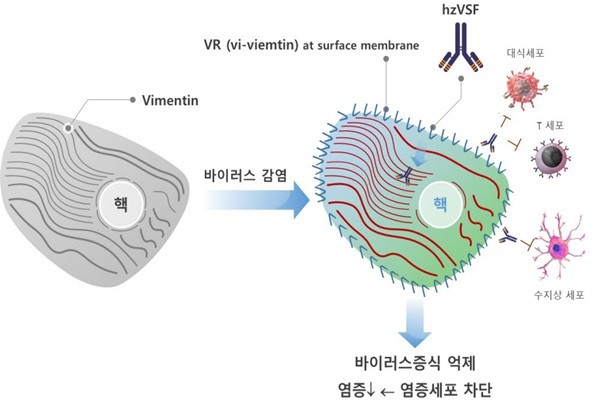
hzVSF was first discovered by CEO Kim and is currently being developed by ImmuneMed as a candidate for COVID-19 treatment. hzVSF comes from humanizing virus suppressing factor (VSF) separated from mouse infected with COVID-19. Protein called vimentin causes a structural change of an infected cell and becomes exposed to the outside of cell membrane. hzVSF is combined with vimentin and causes antiviral activity and suppresses COVID-19 from multiplying. Because cells with immunity do not recognize invasion of a virus, hzVSF can also suppress inflammation that may occur due to immune reaction.
hzVSF prevents virus from multiplying through a biphasic effect of antivirus and anti-inflammation. At the same time, it will suppress inflammation that may occur due to overreaction of the immune system and be helpful for serious COVID-19 patients. Because hzVSF attacks infected cells rather than the virus itself, it can also be used for illnesses caused by other viruses. ImmuneMed initially begun developing a medicine in order to treat patients with hepatitis B and pneumonia caused by influenza.
ImmuneMed became the first South Korean company to receive an approval from the MFDS to use hzVSF to treat COVID-19. hzVSF was then used for seven patients. After hzVSF was administered, it was shown that levels of C-reactive protein and various inflammatory cytokines dropped noticeably. ImmuneMed along with Seoul National University Hospital published a paper on the finding.
“Because steroidal drug that is administered along with antiviral drug generally lowers immunity of COVID-19 patients, there were times when COVID-19 patients suffered from complications even when they became free from COVID-19.” said CEO Kim. “hzVSF can be effective for serious patients with complications and ease further complications.”
Staff Reporter Jung, Hyeonjung | iam@etnews.com